Tumor is one of the main killers that threatens human’s life. In addition to the three conventional treatment methods: surgery, radiotherapy, and chemotherapy, precision treatment technologies such as targeting medicines, immunotherapy, and minimally invasive interventional therapy are widely used in clinical practice. Among them, minimally invasive tumor interventional technic is becoming more and more popular.
Minimally invasive interventional techniques for tumors are divided into vascular interventional therapy and non-vascular interventional therapy. Commonly used non-vascular interventional therapies for solid tumors include heating techniques - microwave or radiofrequency ablation; freezing techniques - Cryoablation; internal irradiation techniques - Iodine implantation; and non-thermal techniques - Nanoknife ablation. Recently, Liang Bing, Vice President of Fuda, shared the clinical application of Nanoknife in tumor ablation.

Nanoknife ablation, also known as irreversible electroporation (IRE), is a non-thermal ablation technology. Nanoknife places an ablation probe into lesion under CT or ultrasound guidance, then uses short-pulse, high-voltage direct current technology to generate microsecond-level electrical pulses, which would form multiple nanoscale irreversible electroporations on the tumor cell’s membrane, destroying the balance inside and outside the cell, leading to cell apoptosis.
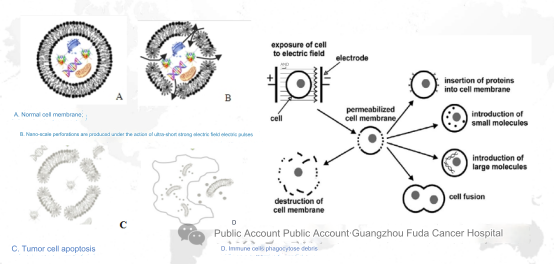
Mechanism:
a. Apply a high-voltage electric field to the phospholipid bilayer of the cell membrane in the form of microsecond and millisecond pulses to generate an unstable electric potential and create two forms of nanoscale pores on the cell membrane;
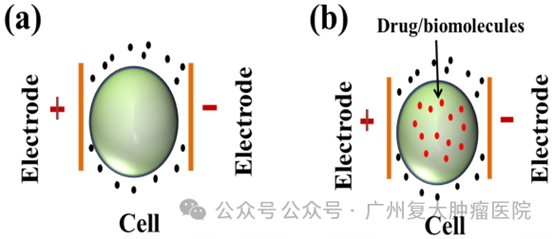
b. Reversible electroporation (RE): Cells can be completely repaired and survive after a certain degree of nanoscale pore damage, which is the so-called reversible electroporation, such as the introduction of intracellular cytotoxic drugs or bleomycin;
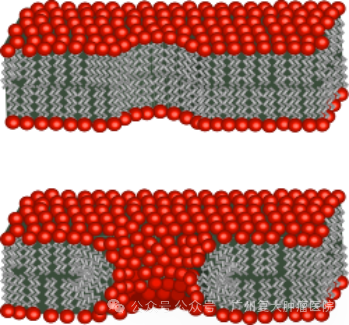
c. Nanoknife is also called irreversible electroporation (IRE): irreversible cell membrane damage occurs through electroporation, leading to cell apoptosis. When an electric field exceeding 0.5 V/nm is applied to the resting transmembrane potential, initially water molecules queue up to file through the hydrophobic center of the bilayer lipid membrane and enter the cell. Later, as the channel length and diameter extending to the hydrophilic group, water molecules quickly enter the cells in large numbers.
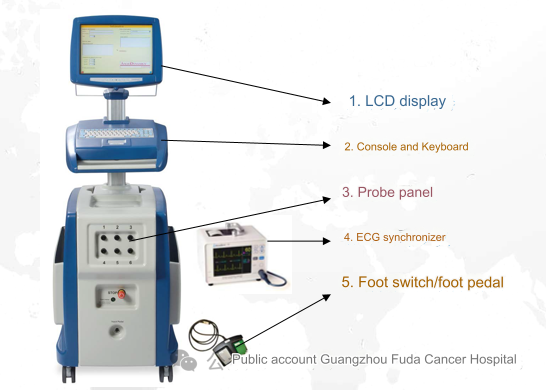
Nanoknife equipment mainly consists of LCD display, console and keyboard, foot switch, ECG synchronizer, and electrode probes. Among them, the ECG synchronizer is mainly used for real-time monitoring of patients' vital signs during ablation.
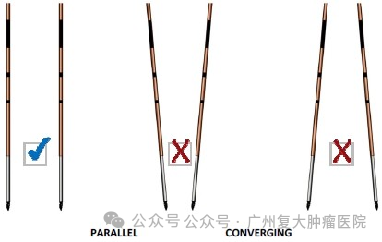
It is worth noting that the conditions that cause the cell membrane to be reversible/irreversible include electric field strengths such as 50-1000v/cm (reversible), 1000-3000v/cm (irreversible), and pulse widths of 70-100 µs. During ablation, at least 2 or more probes are required to work. The needles should be as parallel as possible, and the depth of needle insertion should be kept consistent. The exposure depth of the ablation probe should be reasonably adjusted and the distance between needles should be accurately measured.
In addition, this technology does not generate any heat in the electric field during the ablation process. The nanoknife only targets the lipid bilayer of the cell membrane and has no effect on other molecules such as membrane proteins. The resulting cell apoptosis only occurs in the ablation area, short ablation time, important tissues in the ablation area are preserved, not affected by the heat sink effect, thorough ablation, and the ablation boundary is clear. At the same time, its working principle is to induce cell apoptosis rather than protein denaturation and necrosis, which can better retain tumor antigens, activating anti-tumor immunity. It is said that IRE combined with immunotherapy is expected to improve the long-term efficacy of patients.
Compared with other physical ablation, nanoknife has unique advantages. However, it also has certain shortcomings. For example, strong electricity will cause muscle contraction, so general anesthesia is currently required during the operation. For patients with an implanted pacemaker or defibrillator in the ablation area, electronic equipment and metal parts implanted near the ablation area, the ablation area being the eyes (including eyelids), a history of epilepsy or arrhythmia, and recent episodes of Patients who have had myocardial infarction are not suitable for this treatment.
Nanoknife is widely used in the treatment of tumors in the prostate, liver, pancreas, kidney and other parts of the body due to selectively protects tissue structures such as bronchial walls, blood vessels, bile ducts, pancreatic ducts and nerves that lack phospholipid bilayers and is not affected by the heat sink effect. It has unique advantages especially for tumors in complex locations such as the hepatic hilar area, gallbladder bile duct, pancreas, and ureter. Clinically, it has been found that nanoknife combined with chemotherapy, radiotherapy, immunotherapy, etc. has achieved good results in the treatment of tumors such as pancreatic cancer. Of course, the relevant mechanisms, theories, optimal parameters and imaging monitoring methods, as well as the safety and efficacy of the treatment, need further research.
In 2015, Guangzhou Fuda Cancer Hospital already introduce d nanoknife technology in tumor treatment. On July 1, 2015, under the leadership of President Niu Lizhi, Fuda medical team completed first the nanoknife ablation for pancreatic cancer. At present, our hospital has published nearly 50 research papers related to nanoknife, including more than 20 SCI papers, and published the domestic nanoknife ablation book "New Technology for Cancer Ablation - Irreversible Electroporation".

With the continuous development of medical technology, "micro-nanoknife", as the latest generation of IRE technology, makes IRE tumor ablation treatment more efficient and convenient, and is expected to become a new choice for the treatment of malignant tumors. Currently, Fuda has launched relevant clinical trials.