The emergence of immunotherapy has brought hope to more cancer patients. However, the occurrence of immune-related adverse events ( irAEs ) cannot be ignored. They can affect almost all organ systems, limit the benefits of clinical drugs, and even endanger the patient's life in severe cases. So what should we do in the face of adverse reactions? Recently, Xing Yanli, deputy director of the Fourth Medical Department of Guangzhou Fuda Cancer Hospital, shared the content of immunotherapy-related adverse reaction management.

What are irAEs ?
Immune checkpoint inhibitors (ICIs, mainly including PD-1 inhibitors, PD-L1 inhibitors and CTLA-4 inhibitors) block T cell negative regulatory signals, relieve immunosuppression, and enhance the anti-tumor effect of T cells. At the same time, they may also abnormally enhance their own normal immune response, leading to an imbalance in immune tolerance. When involving normal tissues, they manifest autoimmune-like inflammatory responses, which are called irAEs .
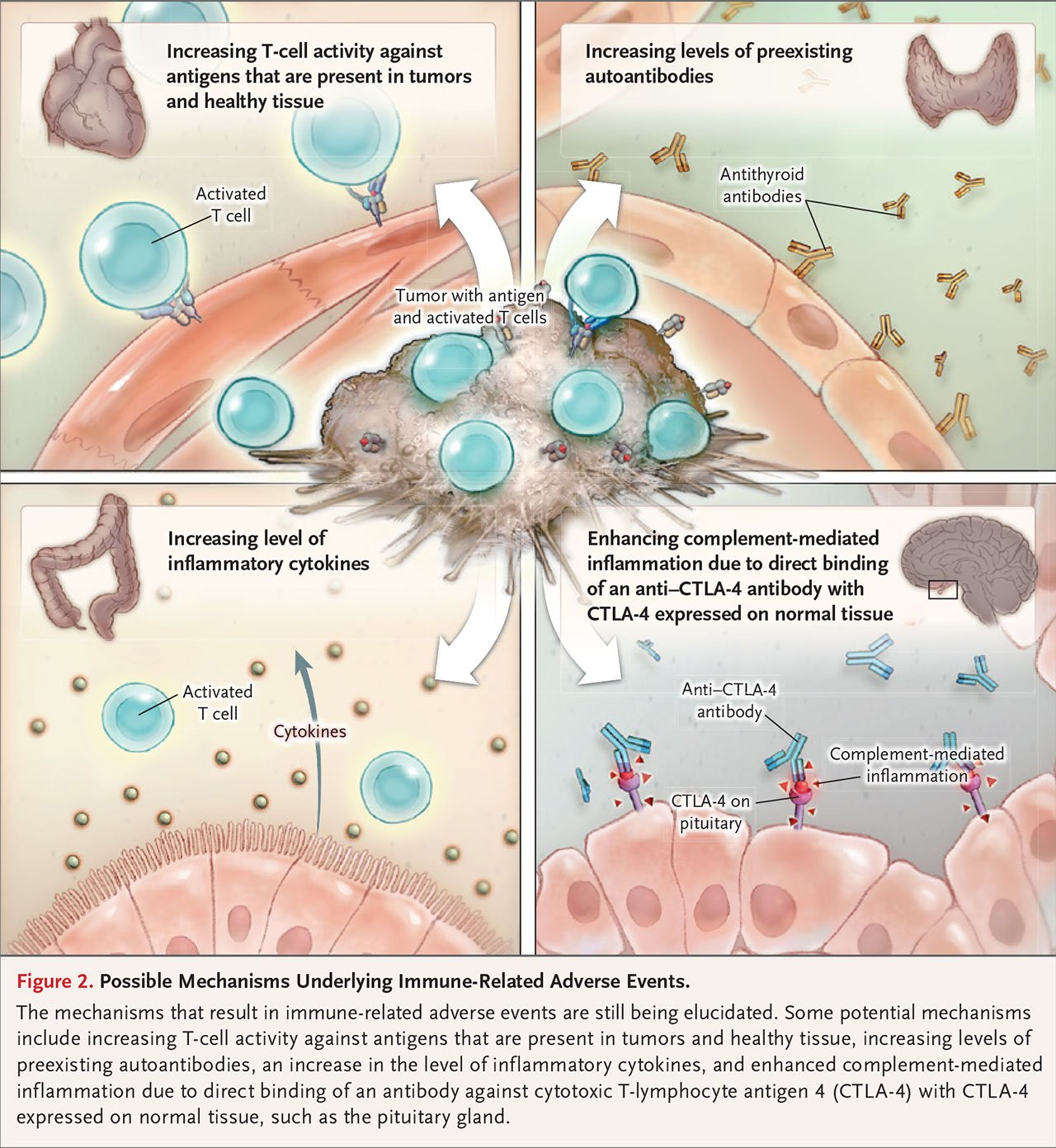
of irAEs ?
caused by abnormal immune mechanisms after the use of immune checkpoint inhibitors , but the exact mechanism is not yet clear. Different from the adverse reactions of traditional treatments (such as chemotherapy and targeted drug therapy ), irAEs have distinct characteristics:
irAEs can involve various organs or systems throughout the body, including the skin, colon, endocrine organs, liver, and lungs, among which the skin and gastrointestinal tract are most commonly affected.
Common irAEs : rash, colitis, hypophysitis, thyroiditis, arthritis, uveitis, anemia, etc.
Life-threatening irAEs : myocarditis, pneumonitis, encephalitis, and hepatitis.
Immunotherapy (IO) has different mechanisms from chemotherapy and targeted therapy, resulting in different adverse reaction spectra. In addition, the incidence and toxicity spectrum of adverse reactions related to different immune checkpoint inhibitors are different. For example, the incidence of adverse reactions of CTLA-4 inhibitors is as high as over 90%, with colitis and hypophysitis being more common , while the incidence of adverse reactions of PD-L1/PD-1 inhibitors is about 70%, with pneumonia, myalgia, and hypothyroidism being more common .
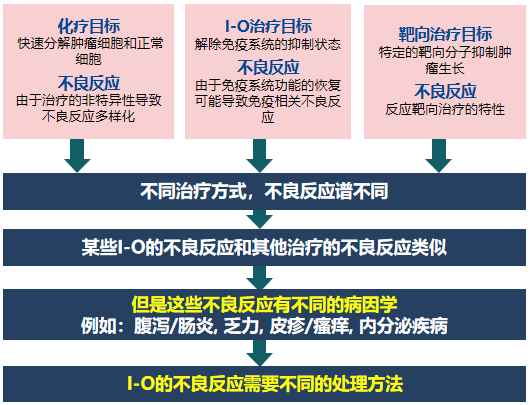
Compared with AEs caused by chemotherapy, the overall incidence of irAEs is lower and the tolerability is better.
![]()
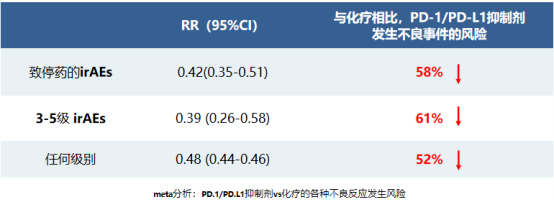
▲This meta-analysis included 20 randomized controlled studies, 10,794 patients, and 5 different tumors
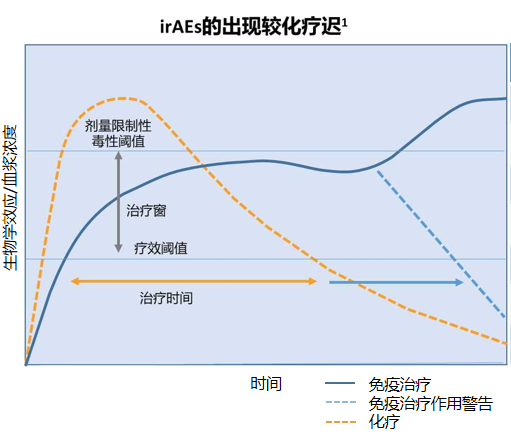
irAEs varies, mostly occurring within 1 to 3 months of immunotherapy, and can be delayed. The adverse reactions caused by combined therapy often occur earlier than those caused by monotherapy. The overall incidence of adverse events related to monotherapy is low, and grade 3 and above adverse events are also lower than those in the combined therapy group.![]()
IrAEs varies in different tumors .
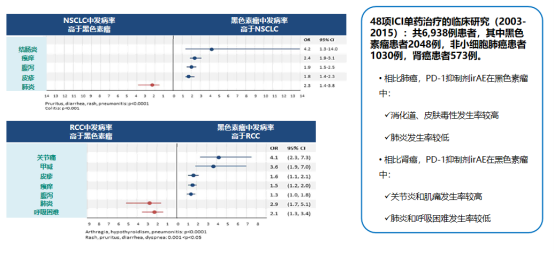
![]()
Different irAEs occur at different times, and most are reversible. For example, enteritis, hepatotoxicity, and pneumonia are relieved quickly, and endocrine-related toxicity is mostly irreversible, but patients can recover through the supplementation of physiological hormones, and immunotherapy can be considered again.
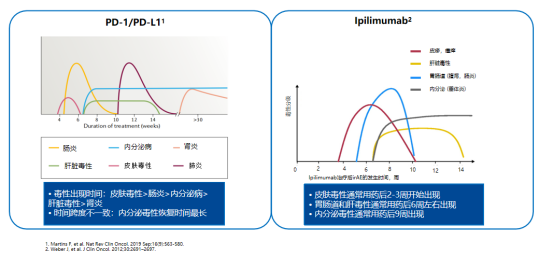
How to deal with irAEs ?
irAEs can occur at any time, even after treatment. Most irAEs are mild to moderate, and life-threatening irAEs occasionally occur . In addition, due to the non-characteristic symptoms and lack of diagnostic criteria, early diagnosis is often difficult. Therefore, in order for immunotherapy to achieve greater clinical benefits, we need to understand immune adverse reactions and achieve early detection, early diagnosis, and early management.
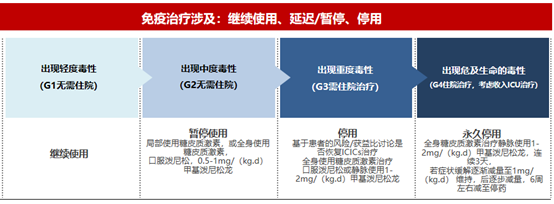
▲ General principles for the treatment of irAEs : Early intervention with glucocorticoids is a key goal in the general management of immune-related toxicity
Common adverse reactions and treatment methods
Skin toxicity
Common skin adverse reactions include rash, itching and vitiligo; less common are alopecia areata, stomatitis, dry skin, etc.; there are also reports of psoriasis exacerbation and psoriasis-like or lichen-like skin in patients with no history of skin diseases. The diagnosis of skin toxicity mainly depends on the patient's medication history and clinical manifestations, such as:
When patients experience adverse skin reactions, other causes, such as infection, reactions to other drugs, etc., must be ruled out first.
Then conduct a comprehensive examination of the skin and mucous membranes, assess the general condition, and perform blood cell counts and liver and kidney function tests if necessary.
If a fatal situation occurs, the immune drug should be permanently discontinued and the patient should be hospitalized with dermatology assistance for treatment.
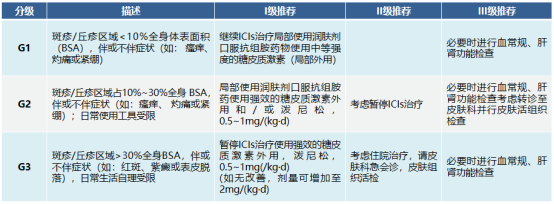
Gastrointestinal (diarrhea/colitis) toxicity
If abdominal pain and diarrhea occur, be alert to the possibility of immune-related gastrointestinal toxicity. Patients with malignant tumors of the digestive system should consider gastrointestinal symptoms caused by the primary disease.

Endocrine system toxicity
Consider hypothyroidism: unexplained fatigue, weight gain, hair loss, intolerance to cold, constipation, depression, and other symptoms;
Consider hyperthyroidism: unexplained palpitations, sweating, increased eating and bowel movements, and weight loss;
Consider hypophysitis in the setting of unexplained persistent headache and/or visual disturbances.

Hepatotoxicity
The main manifestations are increased alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST), with or without increased bilirubin, which often occurs 8 to 12 weeks after the first use of ICIs.
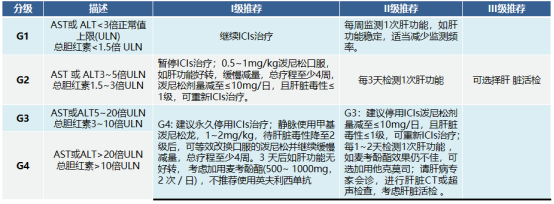
Pulmonary toxicity (pneumonia)
The main symptoms are dyspnea (53%), cough (35%), fever (12%), and chest pain (7%), which occur relatively late. More than 85% of patients can be relieved or cured by discontinuation of medication and immunosuppressive therapy. NSCLC patients with positive driver gene sensitive mutations who receive EGFR-TKI combined with ICIs and patients with COPD, pulmonary fibrosis, and active lung infection are at higher risk.
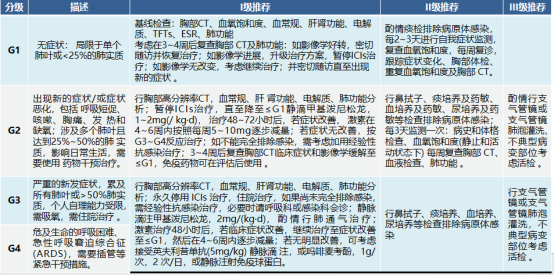
Cardiotoxicity
Symptoms are non-specific, such as chest pain, shortness of breath, pulmonary edema, etc. Once myocarditis is diagnosed, high-dose glucocorticoid treatment is given immediately.
![]()
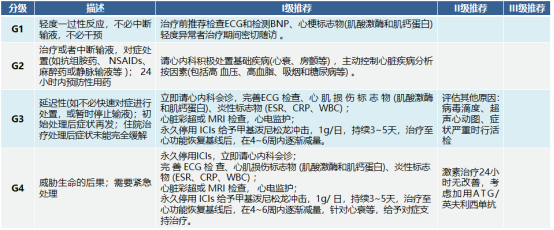
Some studies have shown that the occurrence of rAEs is positively correlated with the efficacy of immunotherapy, and positive clinical outcomes include improved overall response rate (ORR), progression-free survival (PFS), and overall survival (OS). However, the mechanism at the individual patient level, why certain irAEs occur, and the relationship between toxicity and efficacy remain unclear and require more research and verification, which may be used to predict the efficacy of immunotherapy in the future.