Cancer metastasis is the primary cause of cancer-related deaths, accounting for over 90% of such fatalities. Tumor metastasis is a complex process influenced by factors such as plasticity, stress responses, and immune suppression in the tumor microenvironment. Consequently, strategies for treating metastatic tumors are more intricate compared to primary tumors. Recently, Dr. Shijuanjuan, Deputy Director of the Medical Department at Guangzhou Fuda Cancer Hospital, shared insights into the mechanisms of tumor metastasis and innovative treatment strategies.

01 Mechanisms of Tumor Metastasis
Tumor metastasis refers to the process where cancer cells detach from the primary growth site, travel through various routes within the body, proliferate in organs/tissues distant from the primary site, and form secondary tumors. This pathological feature is closely associated with tumor grading and patient prognosis.
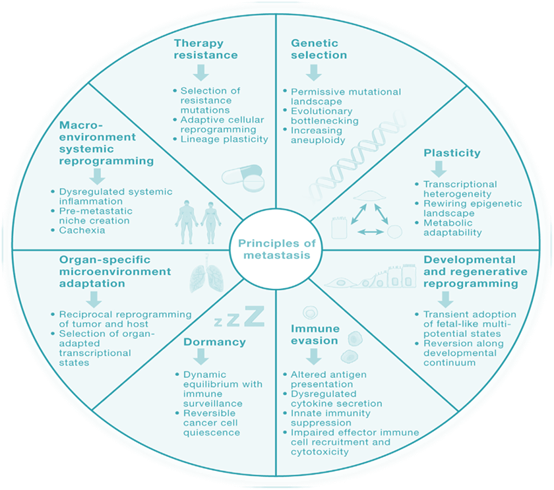
▲ Principles of Tumor Metastasis
Metastasis is a multi-step continuous process resulting from the interaction between tumor cells and the tumor microenvironment (TME), influenced by various factors. Different scholars propose different theories regarding the mechanisms of tumor metastasis.

The metastatic process involves several steps, including the gradual acquisition of increased invasiveness by epithelial cells from the primary malignant tumor. The cells invade the surrounding stroma, disseminate through the bloodstream/lymphatic circulatory system, implant in distant organs, and ultimately proliferate. The key steps in tumor metastasis include:
o Separation of malignant tumor epithelial cells from the basement membrane;
o Invasion of the surrounding stroma, spreading through the bloodstream/lymphatic system, and subsequently settling in distant organs;
o Simultaneously, the primary tumor releases various signaling factors through autocrine, paracrine, or endocrine methods, promoting the formation of a microenvironment conducive to the growth of migrating cells in distant organs;
o The disseminated cancer cells then traverse the capillary/lymphatic vessel walls and enter the parenchyma of distant target organs, where they settle.
Afterward, metastatic tumor cells either enter a dormant state, awaiting suitable signals for awakening, or continue to migrate and settle in distant tissues.
02 Routes and Directions of Tumor Metastasis
Tumor metastasis can occur through various pathways, with the most typical being hematogenous metastasis, lymphatic metastasis, implantation metastasis, and special pathways (including iatrogenic implantation and aerogenous metastasis).

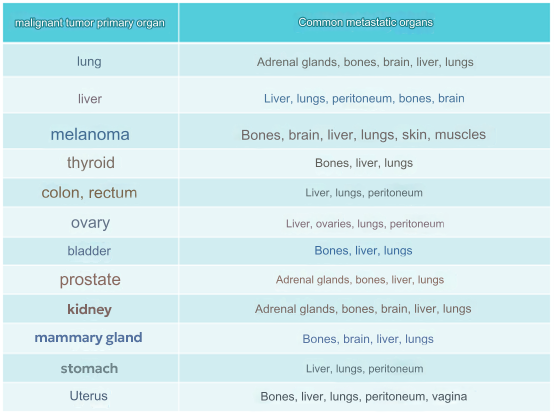
Tumor metastasis is not a random process but exhibits a noticeable targeting of specific organs.
03 Diagnosis and Treatment of Tumor Metastasis
The diagnosis and treatment of tumor metastasis hold significant clinical importance, encompassing the determination of tumor staging, assessment of prognosis, guidance for treatment strategies, monitoring treatment efficacy, and evaluating the risk of recurrence. Various methods are employed in clinical practice for detecting tumor metastasis, including imaging examinations, blood tests, bone marrow aspiration and biopsy, lymph node biopsy, laparoscopy/thoracoscopy, and nuclear medicine examinations.
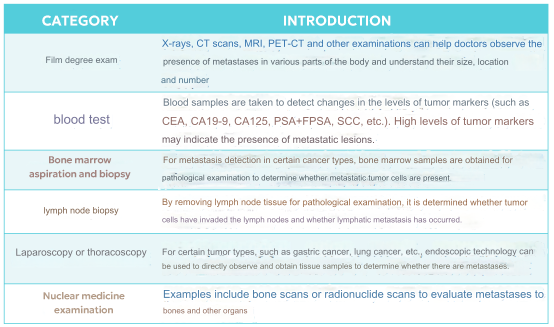
In addition, liquid biopsy, an emerging non-invasive detection technology, was recognized as one of the top breakthrough technologies of 2015 by MIT Technology Review. Currently, popular techniques for the early detection and screening of tumor metastasis involve the detection of trace amounts of circulating tumor cells, circulating abnormal cells (CAC), circulating tumor DNA (ctDNA), exosomes, and other components in the blood.
04 Treatment Strategies for Tumor Metastasis
As a systemic disease affecting organs throughout the body, tumor metastasis currently lacks effective strategies to regulate/intervene in the entire process. Instead, interventions are often limited to specific stages/segments of the metastatic process, such as:
o Direct targeting of the implantation organ where metastatic tumors are located: surgical treatment, radiation therapy;
o Regulation of the metabolism of metastatic tumors: chemotherapy, targeted therapy, immunotherapy.
Although treating tumor metastasis poses numerous challenges, there have been notable advancements in recent years. For instance, in the field of surgical treatment, the use of indocyanine green (ICG) or targeted fluorescent probes, combined with real-time imaging devices, enables the clear presentation of tiny tumor lesions (primary or metastatic) for precise and efficient removal. In targeted therapy, the development of small-molecule drugs with blood-brain barrier (BBB) penetration capabilities effectively reduces the risk of tumors metastasizing to the brain.
With a deeper understanding of the mechanisms of tumor metastasis, new treatment strategies and research directions bring hope for overcoming this challenging aspect of cancer treatment. Examples include:
✍ Targeting Phenotypic Plasticity for Treating Tumor Metastasis
Phenotypic plasticity is a relatively new hypothesis concerning tumor metastasis, referring to the phenotypic differences among tumor cells with the same genotype due to changes in environmental conditions. This reflects the adaptive responses of tumor cells to changes in the environment (primary site vs. metastatic site) to ensure survival and proliferation. Signaling pathways such as TGF-β, Wnt, Sonic Hedgehog (SHH), and Notch, associated with phenotypic plasticity, have been confirmed to influence the process of tumor metastasis. Small molecule inhibitors, monoclonal antibodies, and antibody-drug conjugates (ADCs) developed against these signaling pathways hold promise for breakthroughs in treating tumor metastasis, especially ADCs with potential "combination" capabilities.
Furthermore, in tumor metastasis, heterogeneity exists within the same tumor's primary site or between the primary and metastatic sites. Large-scale genomics studies indicate that this phenomenon is regulated by the epigenome. Thus, intervening in the epigenetic regulation of phenotypic plasticity is expected to have therapeutic effects on treating tumor metastasis.
✍ Immunotherapy for Treating Tumor Metastasis
Immunotherapy, represented by immune checkpoint inhibitors such as PD-1/PD-L1 and CTLA-4, has changed the landscape of cancer treatment and is a popular method for treating tumor metastasis. In addition to PD-1/PD-L1 and CTLA-4, immune therapies targeting other immune checkpoints such as TIM3, LAG3, TIGIT, and B7-H3 are actively being developed.
In tumor metastasis, disseminated tumor cells (DTC) must overcome immune surveillance and attack to successfully settle in a new environment. However, T cell and NK cell exhaustion is widespread in metastatic tumors. Enhancing the activity, survival time, specific targeting, and killing function of immune cells, and combining immune cell therapy with other immune treatment methods, may further improve treatment efficacy.
Tumor metastasis is a complex, systemic disease influenced by multiple factors and remains a major cause of cancer patient mortality. Although there are still many core issues to be addressed in understanding the mechanisms of tumor metastasis, significant progress has been made in the study of heterogeneity and dynamics in the metastasis process. Particularly, insights into epigenetic regulation, the tumor microenvironment, and immune regulation offer hope for breakthroughs in new treatment methods and drugs for tumor metastasis through interventions at critical nodes.