"This thyroid tumor is relatively large, with rich blood supply, and is close to surrounding vascular structures, making surgery somewhat risky. However, we can guide the procedure using ultrasound, avoid the rich nerves and blood vessels in the neck, and insert a 'needle' into the tumor to use 'heat' to destroy the tumor." This "heat" ablation, or microwave ablation, is a method in which we use a "mobile ablation" technique to divide the large tumor into smaller sections for separate ablation. Like a sculptor carving, this allows us to eliminate even the edges and corners thoroughly.
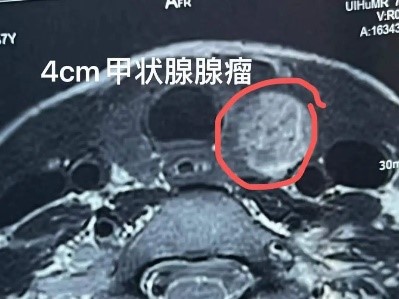
Ultrasound-guided local ablation for thyroid disease has become a focal point of clinical research in recent years due to its minimally invasive nature, safety, and clear therapeutic outcomes, bringing new developments to thyroid disease treatment. Initially, when local ablation techniques were applied to thyroid nodules, the method involved fixed-needle ablation, using one or more fixed needles to cover the tumor with ablation points.
Thyroid lesions are often irregular in shape, and fixed-needle ablation can only expand the ablation zone by extending the duration of action, which doesn't change the shape of the ablation area. Therefore, this method often fails to adapt well, which may explain the high rate of incomplete ablation in fixed-needle groups. Incomplete ablation may result in insufficient reduction of the nodule, with remaining pathological tissue around the lesion potentially leading to proliferative growth.
The multi-needle, multi-point approach not only increases the number of needle insertions, which raises the risk, but also cannot achieve fully adapted ablation during treatment. This is because the thyroid is located near many vital structures (such as the trachea and nerves), and extending the duration of the fixed needle's action is particularly dangerous to these critical tissues.
In 2008, Jeong et al. first proposed the "moving-shot technique" in thyroid radiofrequency ablation. This technique uses mobile ablation to form multiple ablation units of different sizes by moving the needle tip to cover the entire lesion. Subsequent clinical studies have shown positive outcomes. Lim et al. followed up for an average of (49.4 ± 13.6) months, with a mean reduction rate of (93.4 ± 11.7)%. Compared to fixed needle ablation, mobile ablation offers greater flexibility, as the size of the ablation units can be adjusted by changing the speed of needle movement.
For areas near critical structures, the speed of movement can be increased, and smaller ablation units can be used to cover the area. For areas with rich blood supply, the power can be increased, and the movement speed slowed, or even switched to fixed ablation to ensure thorough tissue necrosis, ensuring both safety and long-term therapeutic efficacy.
With the continuous advancement of medical technology, "mobile ablation" is now not limited to thyroid lesions. For example, for an elderly pancreatic cancer patient with a tumor near 6 cm, surrounded by blood vessels and deemed inoperable, I used a combination of chemotherapy and NanoKnife ablation. During the procedure, the NanoKnife needle divided the tumor into segments, using "mobile ablation" to gradually destroy the entire tumor. The ablation was successful, inactivating the tumor while minimizing damage to the body.
Another example involves a patient with metastatic liver cancer. The tumor was located near the diaphragm and adjacent to blood vessels, making thermal ablation risky. Therefore, we opted for NanoKnife ablation, which is less damaging to normal tissues. Due to the irregular growth of the tumor, measuring around 7 cm, we had to conduct "mobile ablation" in regions to eliminate all edges and corners of the lesion as thoroughly as possible.